1. The European landscape of listed biotech companies: definitions, selection, general overview
Like in a clinical trial, our selection globally meets some inclusion and exclusion criteria, with few exceptions. We tried to build a list of companies to have a relatively homogeneous group, or the least heterogenous to be more accurate, and limit the number of outliers. These criteria can be found below. We reviewed the activity of all the companies, and checked the consistency of their business model with the above-listed criteria.
Inclusion criteria:
• Inception in one of the main European countries: France, Germany, UK, Italy, Spain, Belgium, Netherlands, Switzerland, Denmark, Sweden, Norway, Finland, Ireland
• Primary listing on (at least) one of the main European stock exchanges, including the following:
- Euronext, Main market & Euronext Growth: Paris (France), Brussels (Belgium), Amsterdam (Netherlands)
- Deutsche Börse XETRA: Frankfurt (Germany)
- SIX Swiss Exchange: Zurich (Switzerland)
- London Stock Exchange & its Alternative Investment Market/AIM: London (UK)
- Nasdaq OMX - Nordic List & First North: Stockholm (Sweden)
- Oslo Børs: Oslo (Norway)
- Bolsa de Madrid: Madrid (Spain)
- Borsa Italiana: Milan (Italy)
• Companies developing therapeutics, with a focus on the development of their innovative pipeline, including some “specialty” companies, and some companies developing generics/biosimilars (broad “biotech” definition engulfing both “biotech” and “pharmaceuticals” as per their original definitions, called “biotech” for simplicity's sake)
• Companies whose market capitalization/enterprise value essentially relies on their pipeline, or in a mix of their pipeline and their product sales/royalties and not only on sales/profits
• “Large” biotech companies without a fully-owned product at the commercial stage, not yet vertically-integrated
Exclusion criteria:
• European companies directly listed outside Europe (basically on Nasdaq, New York, US), without any secondary listing on the main European markets
• Foreign companies (for which Europe is not considered as their “domestic” territory)
• Pharma companies or pharma-like vertically-integrated companies (valuation with a PER, focus on sales/external growth, or limited innovation in the pipeline)
• Medtech/Diagnostics companies (non-core activity for some companies in our selection)
• Service companies: CROs, CMOs, Drug Discovery companies (very few exceptions for this particular activity), API providers, etc…
• Biotech Funds
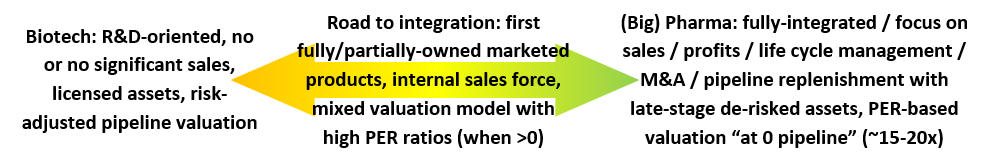
All the companies included in our selection (see Table 1) are at various stages of development for their drug candidate(s). While some companies might remain on the “biotech” side forever, others might become partially or fully-integrated “pharma” companies (depending on manufacturing integration). On the other way, integrated companies might be forced to become “biotech” again if their pipeline was not filled adequately. Therefore, both the position and the direction within the “biotech-to-pharma” transition process (represented just below) mattered for our selection.
However, our selection includes some outliers or special cases, either not meeting the afore-mentioned criteria, or subject to discussion. The most relevant cases can be found below (list not comprehensive):
• Oncodesign (France): another drug discovery company, much smaller than Evotec and with a slightly narrower scope of services compared Evotec but also converging towards an integrated drug discovery model, leveraging its kinase inhibitor platform at the same time. It is basically selected for historical reason;
• Nanobiotix (France): some might consider this company as a medtech (their lead product was regulated as a medical device for their first marketing authorization in Europe), however their model is the one of a biotech, and their product is aimed for a therapeutic use (the regulatory pathway in the US remains to be defined);
• Cosmo (Italy): a specialty pharma focused on GI diseases with therapeutics & medical devices on the market, still with many drivers in the pipeline. The US patent of their lead UC drug will fall next year. We thought it was relevant to have it included in the pool;
• Vectura (UK): a company specialized in COPD/asthma treatment, developing both formulations (CS/LAMA/LABA) and medical devices (inhalers) with partners, included in our selection but under watch as the business model is shifting towards a “specialty CDMO”;
• Indivior (UK): a leading company specialized in addiction and more specifically of opioid addiction, de-merged from Reckitt Benckiser Pharmaceuticals in 2014. It can also be considered as a specialty pharma but contrary to Cosmo, the patent cliff for their leading drug is happening right now, which forced the company to reshape its long-term strategy and portfolio. We thought it was legitimate to have it into our selection;
• Oxford Biomedica (UK): a company with mixed model consisting in manufacturing services & supplying viral vectors for cell and gene therapies, along with developing both an internal pipeline and supporting a partnered pipeline (mainly gene therapies with 1 CAR-T program). Given that the valuation is not (yet) massively dominated by the service segment, we feel that the company is still a relevant pick in our selection;
• Allergy Therapeutics (UK): the company name speaks for itself. The company already has several products on the market (some only on a Named-patient basis) but still has a decent R&D pipeline to be included in our review;
• Basilea (Switzerland): spin-off from Roche in 2000, it is also a commercial stage company that was originally focused in infectious diseases. And like many players in this field, the company has broadened its scope with the addition of oncology assets, with some innovation pending in the pipeline and therefore justifying the presence in our selection;
• PharmaMar (Spain): a conglomerate that is definitely turning into a pharma-only business model, particularly in oncology, with the divestment of 2 group subsidiaries (consumer chemicals in 2018 and cleaning, cleansing, disinfecting products for the industry very recently in 2019). The company has 2 oncology drugs on the market but only 1 available in the US and Europe. We also judged it was a fit in our selection.
• Biotest (Germany): the company specialized in plasma-derived products/IVIg is in complete transformation at several stages, after a strategic refocus initiated in 2015 and a change of control of the company taking place 2 years ago. It’s now in the hands of Creat Group from China via its German subsidiary Tiancheng, but it was not de-listed because of a 2-level share scheme (Voting rights majority via Preferential Shares). This takeover led them to divest their US business to comply with antitrust rules. The company is also about to be brought in kind by Creat as part of a capital increase into Shanghai Raas. Biotest is looking to divest their assets outside plasma products (mainly 3 biologics). In anticipation of the completion of all these changes, Biotest is not included in this selection;
• Puretech Health (UK): a very atypical profile, as it is basically a VC fund with an internal pipeline (we cover only this internal pipeline on our service). Given that it is far from being predominant in the group valuation at this stage, we do not include the company in our selection. Also, some would argue that the company mostly operates in the US but still, it was incorporated in the UK, and the company is only listed in London (for now);
• Biotech Pharmacon (Norway): a specialty pharma company whose most of the business relies on its API supplier and animal health businesses. The company has a small commercial product in human health and a cancer vaccine adjuvant in oncology, but considered as non-core, so it is not included in the review (human therapeutic programs covered on our service);
• Sanochemia (Austria): another specialty pharma (not covered), listed on XETRA in Germany, mainly operating as a CMO, but with a partnered product in back pain, once again considered as non-core.
| AB Science | FR | Acacia Pharma | UK | A1M Pharma | SE | NOXXON Pharma | DE |
| ABIVAX | FR | 4D Pharma | UK | Active Biotech | SE | Probiodrug | DE |
| Adocia | FR | Allergy Therapeutics | UK | Alligator Bioscience | SE | 4SC | DE |
| Advicenne | FR | Avacta | UK | Annexin Pharma. | SE | Biofrontera | DE |
| Biophytis | FR | Destiny Pharma | UK | Asarina Pharma | SE | Cytotools | DE |
| Cellectis | FR | Diurnal Group | UK | Alzecure Pharma | SE | Evotec | DE |
| Cerenis Therapeutics | FR | Evgen Pharma | UK | BioInvent Internat. | SE | Formycon | DE |
| Crossject | FR | Hemogenyx Pharma. | UK | BioArctic | SE | Heidelberg Pharma | DE |
| DBV Technologies | FR | Immupharma | UK | Calliditas Therapeutics | SE | Medigene | DE |
| Erytech Pharma | FR | Indivior | UK | Camurus | SE | Mologen | DE |
| Genfit | FR | Mereo BioPharma | UK | Cantargia | SE | Morphosys | DE |
| GenSight Biologics | FR | Midatech Pharma | UK | CombiGene | SE | Paion | DE |
| Hybrigenics | FR | Motif Bio | UK | Corline Biomedical | SE | co.don | DE |
| Innate Pharma | FR | N4 Pharma | UK | Cyxone | SE | ||
| Inventiva | FR | Nuformix | UK | Diamyd Medical | SE | GeNeuro | CH |
| Lysogene | FR | Okyo Pharma | UK | Gabather | SE | Genkyotex | CH |
| Medincell | FR | Oxford Biomedica | UK | Hansa Biopharma | SE | Addex Therapeutics | CH |
| Nanobiotix | FR | Realm Therapeutics | UK | Immunicum | SE | Basilea Pharma. | CH |
| Neovacs | FR | Redx Pharma | UK | InDex Pharma | SE | Idorsia | CH |
| Nicox | FR | ReNeuron | UK | Infant Bacterial Ther. | SE | Molecular Partners | CH |
| Oncodesign | FR | Sareum | UK | IRLAB Therapeutics | SE | ObsEva | CH |
| Onxeo | FR | Scancell | UK | Isofol Medical | SE | Polyphor | CH |
| OSE Immunother. | FR | Shield Therapeutics | UK | Kancera | SE | Santhera | CH |
| Pharnext | FR | Silence Therapeutics | UK | Karessa Pharma | SE | ||
| Poxel | FR | Summit Therapeutics | UK | Klaria Pharma | SE | Bavarian Nordic | DK |
| Quantum Genomics | FR | Synairgen | UK | Lidds | SE | Genmab | DK |
| Sensorion | FR | Tiziana Life Sciences | UK | Medivir | SE | Oncology Venture | DK |
| Theranexus | FR | ValiRx | UK | NeuroVive | SE | Orphazyme | DK |
| Transgene | FR | Vectura | UK | Oasmia Pharma. | SE | Zealand Pharma | DK |
| Valneva | FR | Verona Pharma | UK | Oncopeptides | SE | Nuevolution | DK |
| Orexo | SE | Saniona | DK | ||||
| argenx | BE | Amryt Pharma | IE | PledPharma | SE | ||
| ASIT biotech | BE | Promore Pharma | SE | Cassiopea | IT | ||
| Bone Therapeutics | BE | BergenBio | NO | Sprint Bioscience | SE | Cosmo Pharma | IT |
| Celyad | BE | Nordic Nanovector | NO | Vicore Pharma | SE | Newron Pharma. | IT |
| Galapagos | BE | PCI Biotech | NO | XBrane Biopharma | SE | Molmed | IT |
| Mithra Pharm. | BE | Targovax | NO | Xintela | SE | ||
| Oxurion | BE | XSpray Pharma | SE | ||||
| Faron Pharma. | FI | ||||||
| Kiadis Pharma | NL | FIT Biotech | FI | Oryzon Genomics | ES | ||
| Pharming Group | NL | Herantis Pharma | FI | PharmaMar | ES |
Finally, this leaves us with the round count of 150 European biotech companies, from 13 European countries, and listed on stock markets from 8 operators.
Of note, we considered Immupharma as a UK biotech company (this is actually a group with several subsidiaries) but it would not be completely wrong to classify it as a French company either.
If you want to check for more companies outside our “biotech” universe, you can have a look at our European Pharma dashboard here, while our European Biotech dashboard (including select diagnostic companies) can be accessed there.
As a reminder, most of the metrics in this review will be consolidated by country, or by cluster (pooled data from 2 countries).
